Abstract
BACKGROUND
Antigen-mediated proliferation and Bcl-2 mediated survival are key to chronic lymphocytic leukemia (CLL) pathogenesis. Ibrutinib is an oral BTK inhibitor affecting antigen-induced proliferation and cell adhesion/migration whilst venetoclax is a potent, highly selective, orally bioavailable Bcl-2 inhibitor affecting CLL cell survival. The treatment of CLL has been revolutionized by these targeted therapies. Both significantly improve survival in CLL but rarely lead to eradication of detectable minimal residual disease (MRD) when given as single agents. The Bloodwise TAP CLARITY trial combined ibrutinib with venetoclax in order to eradicate detectable CLL with the intention of stopping therapy.
METHODS AND PATIENTS
CLARITY is a Phase II trial combining ibrutinib with venetoclax in 50 patients with relapsed or refractory CLL. After 8 weeks of ibrutinib monotherapy (420mg/day), venetoclax was added first at a dose of 10mg/day with weekly escalations to 20mg, 50mg, 100mg, 200mg to a final dose of 400mg/day. No tumour lysis syndrome (TLS) was seen for the first 3 patients starting at 10mg/day of venetoclax so all subsequent patients began at 20mg/day. The primary end-point of CLARITY was the eradication of MRD (<1 CLL cell in 104 leucocytes or MRD4) in blood and bone marrow after 12 months of combined therapy. Key secondary end-points were MRD eradication after 6 months, response by 2008 IWCLL criteria, and safety. We report here the results of CLARITY at the data lock on 14 May 2018. 54 patients were recruited from May 2016 to November 2017. The median number of prior therapies was 1 (range: 1-6) including FCR or BR in 44/54 (81%) and idelalisib plus rituximab in 11/54 (20%). 10/51 (20%) of patients had 17p del, 13/54 (25%) 11q del (but not 17p), and 40/53 (75%) had unmutated IGVH genes. Four patients discontinued treatment due to ibrutinib-related adverse events in the first 8 weeks - prior to starting venetoclax. As per the protocol these patients were replaced so that, in total, 50 patients received ibrutinib in combination with venetoclax.
RESULTS
There were no cases of clinical tumor lysis syndrome (TLS) and only a single case of biochemical TLS (grade 3) which resolved with treatment. Other side-effects were mild and/or manageable, most commonly neutropenia (3/32 grade 2, 29/32 grade 3/4) or gastro-intestinal events (268/278 grade 1/2, 10/278 grade 3/4).
Two Suspected Unexpected Serious Adverse Reactions (SUSARs) were reported (abdominal pain and pemphigus), 31 Serious Adverse Events (SAEs), and 918 Adverse Events (AEs) (of which 75 were grade 3 or 4) were reported. Notably there were nine grade 3 or 4 infections and 29 events of grade 3 or 4 neutropenia. Thus far all SAEs have resolved with appropriate management, and all patients remained on trial following resolution.
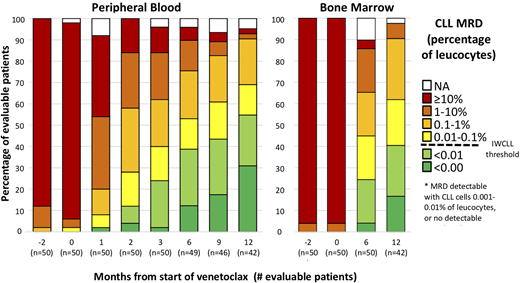
After 6 months of combined ibrutinib plus venetoclax, undetectable MRD was achieved in 19/49 (39%) patients in peripheral blood (PB) and 12/49 (24%) in bone marrow (BM). After 12 months of combined therapy all patients had responded by IWCLL criteria and 23/40 (58%) had achieved a complete remission (CR or CRi). In addition after 12 months of combined therapy 27/31 (87%) have no morphological evidence of CLL in the BM biopsy, 32/34 (94%) had less than 1% CLL cells in the BM aspirate, undetectable MRD (MRD4) was achieved in 23/40 (58%) patients in PB and 17/41 (41%) in BM. There was a continuous improvement in the depth of MRD reduction with 41% of patients achieving less than MRD4 and 29% having undetectable disease by flow cytometry (<1 CLL in 105 leucocytes; MRD5) (see Figure).
CONCLUSIONS
The combination of ibrutinib and venetoclax was well tolerated in patients with relapsed or refractory CLL. Every patient responded and there was a high rate of MRD eradication, in some cases leading to the cessation of therapy.
Hillmen:Pharmacyclics: Research Funding; Gilead Sciences, Inc.: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion Pharmaceuticals, Inc: Consultancy, Honoraria; Acerta: Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Celgene: Research Funding; Novartis: Research Funding. Rawstron:Pharmacyclics: Consultancy, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Beckman Coulter: Research Funding; BD Biosciences: Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brock:GlaxoSmithKline: Equity Ownership; AstraZeneca: Equity Ownership; Lilly: Honoraria; Roche: Other: Reimbursement of expenses; Merck Sharp Dohme: Other: Reimbursement of conference fees. Fegan:Abbvie: Honoraria; Roche: Honoraria; Napp: Honoraria; Gilead Sciences, Inc.: Honoraria; Janssen: Honoraria. McCaig:AbbVie: Membership on an entity's Board of Directors or advisory committees; Gilead: Speakers Bureau. Schuh:Giles, Roche, Janssen, AbbVie: Honoraria. Pettitt:AstraZeneca: Research Funding; Celgene: Research Funding; Gilead: Research Funding; Roche: Research Funding; GSK/Novartis: Research Funding; Napp: Research Funding; Chugai: Research Funding. Gribben:NIH: Research Funding; Novartis: Honoraria; Cancer Research UK: Research Funding; Medical Research Council: Research Funding; TG Therapeutics: Honoraria; Abbvie: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Acerta Pharma: Honoraria, Research Funding; Kite: Honoraria; Unum: Equity Ownership; Wellcome Trust: Research Funding; Pharmacyclics: Honoraria; Roche: Honoraria. Patten:L Hoffman La Roche: Honoraria, Research Funding; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel, Research Funding; AbbVie Inc: Honoraria, Other: travel; Janssen: Honoraria, Other: travel. Devereux:Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: Personal fees; Novartis: Membership on an entity's Board of Directors or advisory committees. Bloor:AbbVie: Research Funding; Janssen: Research Funding. Fox:Roche: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Sunesis: Consultancy; Gilead: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Celgene: Consultancy, Other: travel support, Speakers Bureau; Abbvie: Consultancy, Other: travel support, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: travel support, Speakers Bureau. Forconi:Janssen-Cilag: Consultancy; Abbvie: Consultancy. Munir:Janssen: Honoraria; Abbvie: Honoraria; Gilead: Honoraria; Novartis: Honoraria; Alexion: Honoraria; MorphoSys: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal